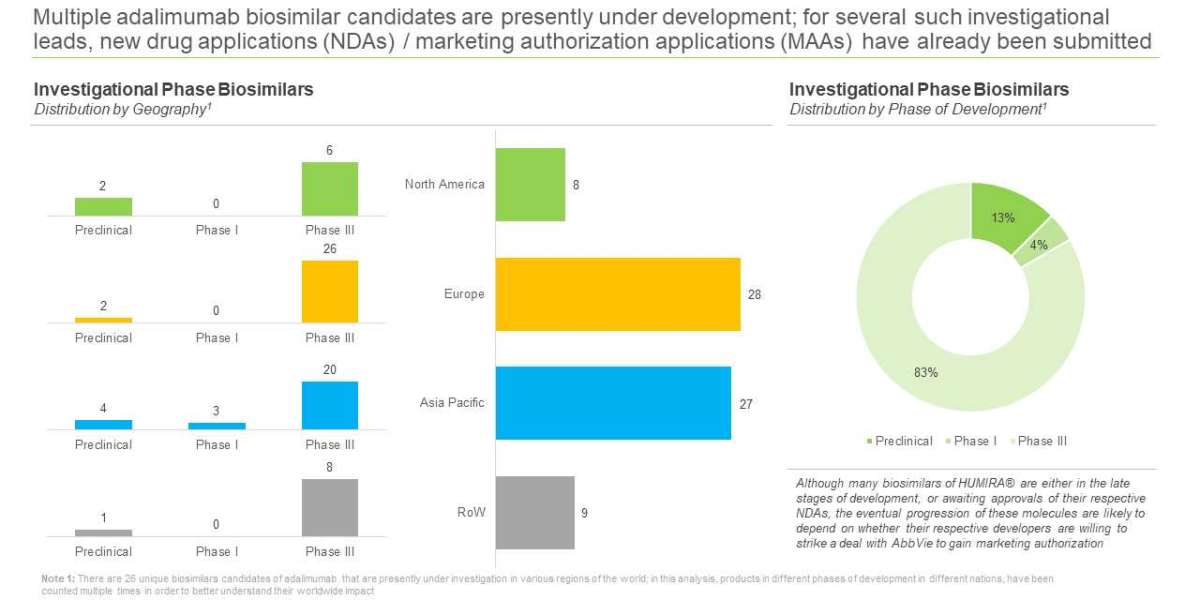
Unlike other biosimilar markets, competition among follow-on products of HUMIRA® is very high and several eager companies are attempting to carve out a share of the multibillion-dollar opportunity associated with this blockbuster product
Roots Analysis has announced the addition of the “HUMIRA® (Adalimumab) Biosimilars – Pipeline Review and Partnerships” report to its list of offerings.
Detailed review of the adalimumab biosimilars pipeline and affiliated developer landscape, featuring a list of involved innovator companies and their respective therapy candidates. It includes insights based on the current status of biosimilar candidates, and important developer related details (including headquarters, and type of developer).
For additional details, please visit
https://www.rootsanalysis.com/reports/humira-biosimilars-pipeline-review-and-partnerships.html
Being one of the first biologics against anti-inflammatory diseases, HUMIRA® is considered an aberration in terms of patent protection, with AbbVie strategically covering novel uses of the product and thereby, enjoys uncharacteristically prolonged marketing exclusivity. In fact, a year after multiple adalimumab biosimilars were launched in Europe, the originator released a statement that its diverse product placement strategy was able to maintain profitability, despite the availability of enemy products.
Key Report Highlights
Despite direct competition from marketed biosimilars, global sales of the blockbuster drug, HUMIRA® increased by 4% in 2020
In the same year, revenues from the sales of the originator product were reported to be responsible for 30% of AbbVie’s earnings from product sales; it is worth mentioning that this is the scenario almost two years after the regulated launch of follow-on products in Europe.
There are close to 50 companies, across the world, claiming to be engaged in the development of HUMIRA® biosimilars
Although multiple companies have obtained regulatory approvals for their respective biosimilars to HUMIRA®, the launch of these products in regulated markets has been delayed until 2023 and is subject to the procurement of the appropriate licenses from AbbVie.
To request a sample copy / brochure of this report, please visit this https://www.rootsanalysis.com/reports/humira-biosimilars-pipeline-review-and-partnerships/request-sample.html
Key Questions Answered
- Who are the key players engaged in the development of biosimilars of adalimumab?
- In which global marketplaces are HUMIRA® biosimilars currently available?
- What is the current scenario within the clinical development landscape of adalimumab biosimilars?
- How many biosimilar development programs have and what were the reasons?
- Who are the key players involved in the commercialization of adalimumab biosimilars across the world?
- What kind of partnerships have been inked between stakeholders in this domain?
The research includes detailed profiles of companies having approved / launched biosimilars across different global regions; each profile features an overview of the company, information related to its current portfolio of adalimumab biosimilars, financial information (if available) and key product related specifications.
- Amgen
- Bio-Thera Solutions
- Boehringer Inglheim
- Cadila
- Celltrion
- CinnaGen
- Cipla
- Emcure
- Fresenius Kabi
- Fujifilm Kyowa Kirin Biologics
- Hetero Biopharma
- Hetero Healthcare
- Innovent Biologics
- LG Chem (formerly LG Life Sciences)
- Pfizer
- Reliance Life Sciences
- Samsung Bioepis
- Sandoz
- Shanghai Henlius Biotech
- Torrent Pharmaceuticals
- Zydus Cadila
- Zydus Research Centre
For additional details, please visit
https://www.rootsanalysis.com/reports/humira-biosimilars-pipeline-review-and-partnerships.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
- Avastin® (Bevacizumab) Biosimilars – Pipeline Review and Partnerships
- Herceptin® (Trastuzumab) Biosimilars – Pipeline Review and Partnerships
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact Information
Roots Analysis Private Limited
Ben Johnson
+1 (415) 800 3415
Facebook - https://www.facebook.com/RootsAnalysis
LinkedIn - https://www.linkedin.com/company/roots-analysis/mycompany/
Twitter - https://twitter.com/RootsAnalysis
Medium - https://medium.com/@RootsAnalysis
Pinterest - https://in.pinterest.com/RootsanalysisPin/_saved/